How Much Baking Soda To Neutralize Sulfuric Acid. Check the pH. So this means 2 moles of sodium bicarbonate is needed to neutralise 1 mole of sulfuric acid.
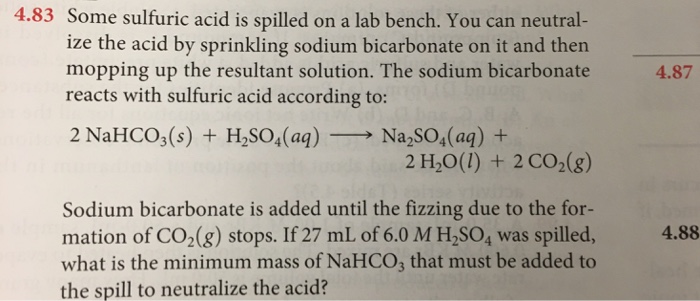
If you have a quantity of concentrated sulphuric acid you can pour it into a solution of sodium hydroxide. So this means 2 moles of sodium bicarbonate is needed to neutralise 1 mole of sulfuric acid. How much baking soda does it take to neutralize sulfuric acid.
2NaHCO3 H2SO4 Na2SO4 2H2O 2CO2 So this means 2 moles of sodium bicarbonate is needed to neutralise 1 mole of sulfuric acid.
Wait until bubblingfizzing has stopped 3. How much baking soda to neutralize sulfuric acid Diluted sulfuric acid 50 concentration or lower is easier to handle. Be careful not to over- neutralize 4. Be careful not to over-neutralize 4.

